Doppler Effect
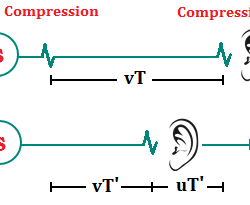
This apparent change in frequency of the wave it due to motion of the source or the observer is called Doppler Effect d. Observer Stationary and Source Moving: Now suppose the observer is at rest with respect to the medium and the source moves towards the observer at a speed u which is less than Read more about Doppler Effect[…]