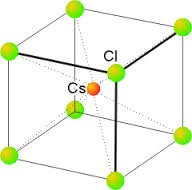
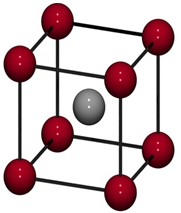
Imperfections in Solids – I
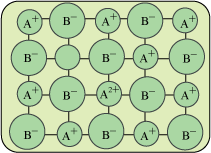
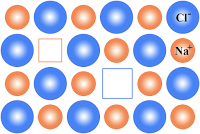
Defects in Non-Stoichiometric Compounds: The compounds in which the ratio between numbers of anions to number of anions is not equal to ratio indicated by the formula. Metal Excess Defect Metal Deficiency Defect. a. Metal Excess Defect: In these compounds there is an excess of cations along with the electrons occurs. 1. Anion Vacancy: ⇒ Read more about Imperfections in Solids – I[…]