Crystal System: There are
230-crystal forms
32-point groups
7- Crystal systems
14- Bravais Lattice
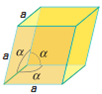
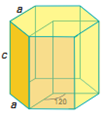
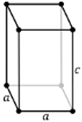
There are seven types of unit cell formed. These are Cubic, Tetragonal, Orthorhombic, Monoclinic, Hexagonal, Rhombohedral or Trigonal and Triclinic. The 7 crystal systems can be remembered as C T O M T R H
|
Crystal System |
Relation between a, b, c, | Relation between α, β, ɣ |
No. of Planes and Axes |
|
Cubic |
a = b = c | α = β = ɣ = 90⁰ |
9 planes + 4 axes |
|
Tetragonal |
a = b ≠ c | α = β = ɣ = 90⁰ |
5 planes + 5 axes |
|
Orthorhombic |
a ≠ b ≠ c | α = β = ɣ = 90⁰ | 3 planes + 3 axes |
|
Monoclinic |
a ≠ b ≠ c | α = β = 90⁰ ≠ ɣ |
1 plane + 1 axis |
|
Triclinic |
a ≠ b ≠ c | α ≠ β ≠ ɣ ≠ 90⁰ |
0 |
|
Rhombohedral |
a = b = c | α = β = ɣ ≠ 90⁰ |
7 planes + 7 axes |
|
Hexagonal |
a = b ≠ c | α = β = 90⁰, ɣ = 120⁰ |
7 planes + 7 axes |
Bravais lattices: The Bravais lattice are the distinct lattice types which when repeated can fill the whole space. In three dimensions there are 14 Bravais lattices
|
Primitive |
Side Centered | Body Centered |
Face Centered |
 |
 |
 |
|
|
Cubic |
|||
|
a ≠ c |
a ≠ c |
||
|
Tetragonal |
|||
a ≠ b ≠ c |
a ≠ b ≠ c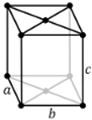 |
a ≠ b ≠ c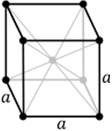 |
a ≠ b ≠ c |
|
Orthorhombic |
|||
|
α ≠ 90⁰, β, γ = 90⁰ |
α ≠ 90⁰, β, γ = 90⁰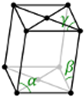 |
||
|
Monoclinic |
|||
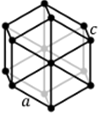 Hexagonal Hexagonal |
α = β = γ ≠ 90⁰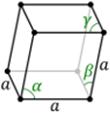 Trigonal Trigonal |
α, β, γ ≠ 90⁰ |
|
|
Crystal |
Description |
Co. Number |
|
Rock Salt (NaCl) |
Cl⁻ ions are in CCP and Na⁺ ions occupy octahedral voids |
Cl⁻ → 6 Na⁺ → 6 |
|
Zincbled (ZnS) |
S²⁻ ions are in CCP Zn²⁺ ions occupied tetrahedral voids. |
Zn²⁺ → 4 S²⁻ → 4 |
|
CsCl |
Cl⁻ ions are in HCP Cs⁺ ions in the body of the cube. |
Cl⁻ → 8 Cs⁺ → 8 |
|
Wurtzrite (ZnS) |
S²⁻ ions are in HCP Zn²⁺ ions occupies tetrahedral void. |
Zn²⁺ → 4 S²⁻ → 4 |
|
Florite (CaF₂) (AB₂) |
Ca²⁺ ions are in CCP F⁻ ions occupies all the tetrahedral voids |
Ca²⁺ → 8 F⁻ → 4 |
|
Anti Florit (A₂B) |
A⁺ ions occupies all the tetrahedral voids, B²⁻ ions are in CCP |
A⁺ → 4 B²⁻ → 8 |
Structure of CsCl:
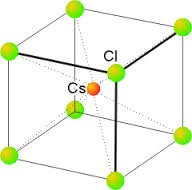
1. Total no: of ions = 9 (1Cs⁻ + 8 Cl⁻ [Or] 1 Cl⁻ + 8 Cs⁺)
2. No: of Cs⁺ per unit cell = 0/8 + 0/4 + 0/2 + 1/1 = 1
3. No: of Cl⁻ per unit cell = 8/8 + 0/4 + 0/2 + 0/1 = 1
4. No: of CsCl formula units per unit cell = 1.
5. Co-ordination number
Each ion is surrounded by eight ions of its counterpart.
So, coordination number for
Cs⁺ → 8
Cl⁻ → 8
6. Body diagonal = √3a = 2 (r Cs⁺ + r Cl⁻)
Structure of NaCl:
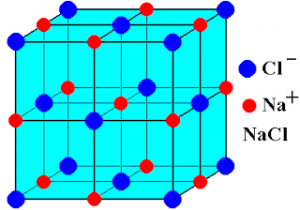
a. Total no: of ions = 27 (13 Na⁺ + 14 Cl⁻ [Or] 14 Na⁺ + 13 Cl⁻)
b. No: of Na⁺ ions per unit cell = 8/8 + 12/4 = 4 {1 is at body centered 12 are at edges}
c. No: of Cl⁻ ions per unit cell = 6/2 + 8/8 = 3 {8 at corners and 6 at face center}
d. No: of formula units per unit cell = 4
e. Co-ordination no: of Na⁺ = 6
f. Cl⁻ = 6
g. Edge length = a = 2 (rNa⁺ + rCl⁻)

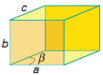
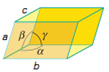
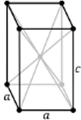
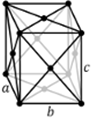
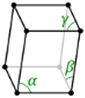
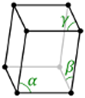 Triclinic
Triclinic