Kirchhoff’s Laws
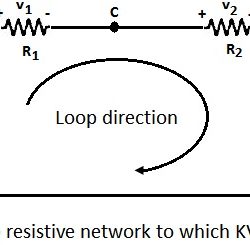
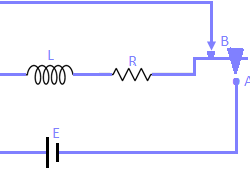
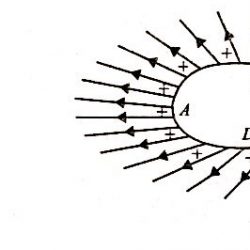
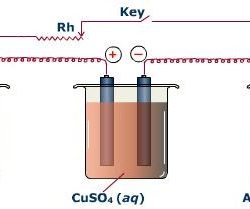
Kirchhoff’s Voltage Law: Kirchhoff’s voltage law states that the algebraic sum of all branch voltages around any closed loop of a network is zero at all instants of time. It is a consequence of the law of conservation of energy. Voltage being the energy (or work) per unit charge. A simple resistive network with voltage Read more about Kirchhoff’s Laws[…]