1. Addition polymerization (or) chain growth polymerization:
⇒ The molecules of the same monomer or different monomers add together on a large scale to form a polymer.
⇒ The monomers used are unsaturated compounds.
Eg: Alkenes, alkadienes and their derivatives.
⇒ Can take place through the formation of either free radicals or ionic species.
Free radical mechanism: A variety of alkenes or dienes and their derivatives are polymerized in the presence of a free radical generating initiator (catalyst) like benzoyl peroxide, acetyl peroxide, tert-butyl peroxide etc.,
Eg: Polymerisation of ethene to polythene.
The free radical mechanism involves following steps.
Chain initiation step:
\(\underset{Benzoyl\,Peroxide\,}{\mathop{{{C}_{6}}{{H}_{5}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-O-O-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-{{C}_{6}}{{H}_{5}}}}\,\xrightarrow{{}}2{{C}_{6}}{{H}_{5}}\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-O\xrightarrow{{}}\underset{Phenyl\,Radical}{\mathop{2\overset{\bullet }{\mathop{{{C}_{6}}}}\,{{H}_{5}}}}\,\).
Ċ₆H₅ – CH₂ = CH₂ → 2C₆H₅ – CH₂ – ĊH₂
Chain propagation step:
C₆H₅ – CH₂ – ĊH₂ + CH₂ = CH₂ → C₆H₅ – CH₂ – CH₂ – CH₂ – ĊH₂ → C₆H₅ (- CH₂ – CH₂-)n – CH₂ – ĊH₂
Chain terminating step: For termination of the long chain, these free radicals can combine in different ways to form polythene.
Eg: C₆H₅ – (- CH₂ – CH₂ -)n – CH₂ – ĊH₂ + C₆H₅ – (- CH₂ – CH₂ -)n – CH₂ – ĊH₂ → C₆H₅ – (- CH₂ – CH₂ -)n – CH₂ – CH₂ – CH₂ – CH₂ – (- CH₂ – CH₂ -)n – C₆H₅
Preparation of some important addition polymers:
a) Polythene: These are of two types.
i) Low density polythene (LDP):-
⇒ By polymerization of ethene
⇒ Temperature: 350 K to 570 K in the presence of traces of dioxygen or peroxide initiator.
⇒ LDP obtained through the free radical addition and H atom abstractions has highly branched structure.
⇒ It is chemically inert and tough but flexible and a poor conductor of electricity.
ii) High density polythene (HDP):-
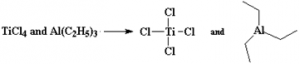
⇒ Addition polymerization of ethene in a hydrocarbon solvent in the presence of Ziegler – Natta Catalyst.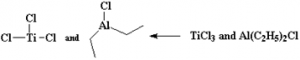 These are two sets of Zieglar – Natta catalyst/ co – catalyst systems. Either way, we have four chlorine atoms.
These are two sets of Zieglar – Natta catalyst/ co – catalyst systems. Either way, we have four chlorine atoms.
 ⇒ Temperature: 333 K to 343 K⇒ Pressure of 6 – 7 atm.
⇒ Temperature: 333 K to 343 K⇒ Pressure of 6 – 7 atm.
⇒ HDP molecules consists linear molecules, its high density is due to close packing. It is also chemically inert and more tough and hard.
⇒ It is used for manufacturing of buckets, bottles, pipes, etc.,
b) Polytetrafluoro ethene (Teflon):
⇒ By heating tetrafluoroethene with a free radical or per sulphate catalyst at high pressure.
⇒ It is chemically inert and resistant to attack by corrosive reagents.
⇒ It is used in making oil seals and gaskets.
\(\underset{Tetrafluoroethene}{\mathop{nC{{F}_{2}}=C{{F}_{2}}}}\,\xrightarrow[High\,\,Pr essure]{Catalyst}\underset{Teflon}{\mathop{{{\left[ -C{{F}_{2}}-C{{F}_{2}}- \right]}_{n}}}}\,\).
c) Polyacrylonitrile:
⇒ The addition polymerization of acrylonitrile in the presence of a peroxide catalyst leads to the formation of polyacryionitrile.
⇒ It is used as a substitute for wool in making commercial fibres as Orlon or acrilan.
\(\underset{Acrylonitrile}{\mathop{nC{{H}_{2}}=CHCN}}\,\xrightarrow[Peroxide\,\,catalyst]{Polymerisation}\underset{Polyacrylonitrile}{\mathop{{{\left[ -C{{H}_{2}}-\overset{CN}{\mathop{\overset{|}{\mathop{C}}\,}}\,H- \right]}_{n}}}}\,\).
2. Condensation polymerization or step Growth polymerization:
⇒ Involves a repetitive condensation reaction between two bifunctional monomers.
⇒ Results in the loss of simple molecules as water, alcohol, etc.
⇒ The product of each step is again a bi functional species and the sequence of condensation goes on.
⇒ So this process is also called as step growth polymerization.
Some important condensation polymerization reactions characterized by their linking units.
Polyamides:
⇒ Possess amide linkages.
⇒ Prepared by condensation polymerization of diamines with dicarboxylic acids and also of amino acids and their lactums.
Nylon 6, 6: Prepared by the condensation polymerization of hexamethylenediamine with adipic acid under high pressure and at high temperature.
\(nHOOC{{\left( C{{H}_{2}} \right)}_{4}}COOH+n{{H}_{2}}N{{\left( C{{H}_{2}} \right)}_{6}}N{{H}_{2}}\xrightarrow[High\,\,pressure]{553K}\underset{Nylon\,6,6}{\mathop{{{\left[ -\overset{H}{\mathop{\overset{|}{\mathop{N}}\,}}\,-{{\left( C{{H}_{2}} \right)}_{6}}-\overset{H}{\mathop{\overset{|}{\mathop{N}}\,}}\,-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-{{\left( C{{H}_{2}} \right)}_{4}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,- \right]}_{n}}}}\,\).
Uses: It is used in making sheets, textile industry.
Nylon 6: Heating caprolactum with water at a high temperature.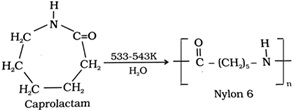 Uses: It is used in the manufacture of tyre cords, fabrics and ropes.
Uses: It is used in the manufacture of tyre cords, fabrics and ropes.
Polyesters: Poly condensation products of dicarboxylic acids and diols.
Eg: Dacron or terylene.
It is manufactured by heating a mixture of ethylene glycol and terephthalic acid at 420 to 460 k in the presence of Zinc acetate antimony trioxide catalyst.![]() Phenol – formaldehyde polymer:
Phenol – formaldehyde polymer:
⇒ These are obtained by condensation reaction of phenol with formaldehyde in the presence of either acid or base catalyst.
⇒ The reaction starts with the initial formation of o – and/ or p – hydroxylmethylphenol derivatives, which further react with phenol to form compounds having rings joined to each other through – CH₂ groups.
⇒ The initial product could be a linear product- Novolac
⇒ Novolac is used in paints.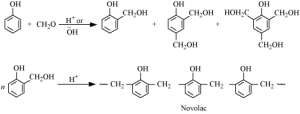 Bakelite: Novolac on heating with formaldehyde undergoes cross linking to form an infusible solid mass called Bakelite.
Bakelite: Novolac on heating with formaldehyde undergoes cross linking to form an infusible solid mass called Bakelite.
Uses: It is used in combs, phonograph records, etc.
Structure: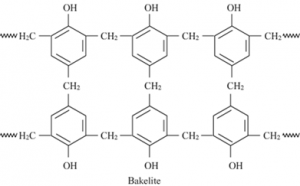 Melamine formaldehyde polymer: Melamine formaldehyde polymer is formed by the condensation polymerization of melamine and formaldehyde.
Melamine formaldehyde polymer: Melamine formaldehyde polymer is formed by the condensation polymerization of melamine and formaldehyde.
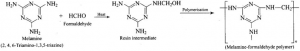 Uses: It is used in the manufacture of unbreakable crockery.
Uses: It is used in the manufacture of unbreakable crockery.
3. Copolymerization:
⇒ A mixture of more than one monomeric species is allowed to polymerize and form a copolymer.
⇒ The copolymer can be made not only by chain growth polymerization but by step growth polymerization also.
Example: Mixture of 1, 3 – butadiene and styrene can form a copolymer.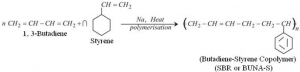 ⇒ Copolymers have properties quite different from homopolymers.
⇒ Copolymers have properties quite different from homopolymers.
⇒ For example, butaiene – styrene copolymer, is quite tough and is a good substitute of natural rubber. It is used in manufacture of auto tyres, footwear components, cable insulation etc.
