Moseley’s law
Soon after Rutherford’s scattering theory had been confirmed by experiment (about 1913), the one-to-one association of an atomic number Z with each element was solidified by the work of Henry Moseley (1887- 1915). He used Bohr’s model of atomic structure to determine the energy emitted when low-level electrons change orbitals. This energy has a strong dependence on atomic number, so that by measuring the energy of the x-rays characteristic of an element, its atomic number Z can be unambiguously determined. In today’s lab you will measure the x-ray spectra of a number of elements and also identify several unknown elements by looking at their characteristic x-ray spectra.
A law that relates the frequency of the spectral lines of the characteristic X radiation of a chemical element to its atomic number. This law was experimentally established by H. Moseley in 1913. According to Moseley’s law, the square root of the frequency v of a spectral line of the characteristic radiation of an element is a linear function of its atomic number Z.
\(\sqrt{\frac{\nu }{R}}\,=\,\frac{Z\,-\,{{S}_{n}}}{n}\)
Where,
R = Rydberg constant,
Sn = Screening constant,
N = Principal quantum number.
On a Moseley plot, the dependence of √v on Z is a series of lines (Such as the Kα lines,
Lα lines, and Mα lines, which correspond to the values n = 1, 2, and 3).
Moseley’s law was incontrovertible proof of the correctness of the arrangement of the elements in D. I. Mendeleev’s periodicsystem of the elements and the law helped to clarify the physical significance of Z.
According to Moseley’s law, the characteristic X – ray spectra do not display the periodic regularities that are inherent in optical spectra.
This indicates that the inner electron shells of the atoms of all elements, which are manifested in the characteristicX-ray spectra, have an analogous structure.
Subsequent experiments revealed some deviations from a linear.
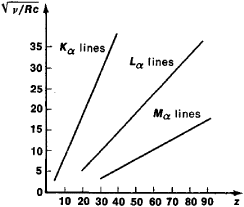
Moseley plot for the Kα lines, Lα lines, and Mα lines of characteristic X – radiation. The atomic number of the element Z is plotted along the axis of abscissas and the quantity √v/RC is plotted along the axis of ordinates.
Dependence for the transition groups of elements: The deviations being due to the change in the order in which the outer electron shells are filled and also for heavy atoms, in which that the velocities of the nucleus and the state of the outer electron shell. The study of these shifts makes it possible to obtain detailed information about the atom.
