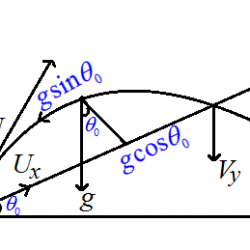
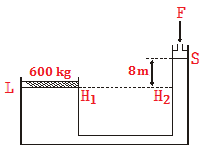
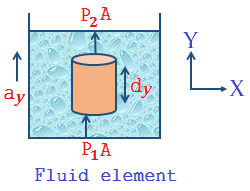
Specific heat capacity
Monatomic gas: Using the law of equiportition of energy the average energy of molecule at temperature Total internal energy of a gas is Cv [Monatomic gas] = For an ideal gas. CP – CV = R CP = J/2 R Ratio of specific heats. Diatomic gases: Total internal energy [law of equiportition of energy] . Read more about Specific heat capacity[…]