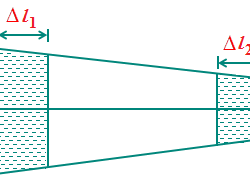
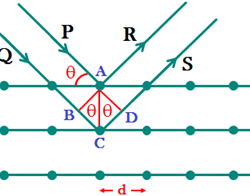
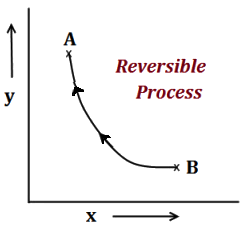
Concentrations of the Solution
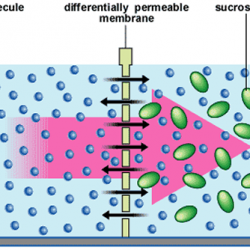
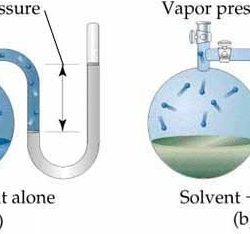
Concentrations of the solution can be expressed as 1. Molarity: No: of moles of solute present in one liter solution. Units: mol L¯¹ Ex: 0.5 M ethanol means 0.5 moles of ethanol dissolved in one liter. 2. Mass percentage (w/w) = Ex: a solution of 20% salt in water by mass means: 20g of salt + Read more about Concentrations of the Solution[…]