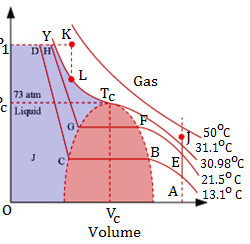
Acids, bases and salts
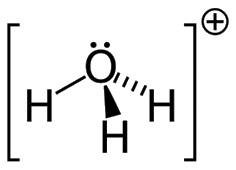
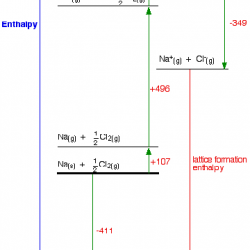
When acids and bases are mixed in the right proportion they react with each other to give salts and water NaOH + HCl ® NaCl + H₂O Arrhenius Concept of Acids and Bases: According to Arrhenius theory, acids are substances that dissociates in water to give hydrogen ions H + (aq) and bases are substances Read more about Acids, bases and salts[…]