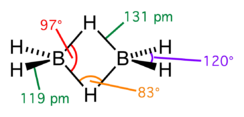
Diborane Structure
Preparation: 4BH3 + 3LiALH4 → 2B2H6 + 3LiF + 3AlF3 2NaBH4 +I2 → B2H6 + 2NaI + H2 2BF3 + 6NaH → B2H6 + 6NaF Structure: Diborane contains four terminal and two bridging hydrogen atoms The terminal H atoms and B atoms lie in one plane. The bridging H atoms lie above and below the plane Properties: Colourless highly toxic gas B.P : 180 K Catches Read more about Diborane Structure[…]