It is also called as oxygenated water (H2O – O)
In its pure form it is a colorless liquid, slightly more viscous than water
Structure:
Hydrogen peroxide (H2O2) is a non-planar molecule. It has half opened book structure. Two oxygen atoms lying on spine of the book with O – O peroxy linkage and 2 ‘H’ atoms on each leaf of book.
The molecular structures of gaseous and crystalline H2O2 are significantly different. This difference is attributed to the effects of hydrogen bonding, which is absent in the gaseous state.
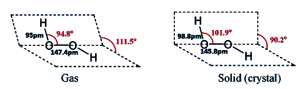 Preparation:
Preparation:
BaO2.8H2O + H2SO4 → H2O2 + BaSO4↓ + 8H2O
Na2O2 + H2SO4 → Na2SO4 + H2O2.
This is Merck’s process.
Hydrogen peroxide is prepared by hydrolysis of the ammonium peroxydisulfate, which was itself obtained via the electrolysis of a solution of ammonium bisulfate (NH4HSO4) in sulfuric acid.
(NH4)2S2O8 + 2 H2O → H2O2 + 2 (NH4) HSO4
Anthraquinone process:
Industrially it is prepared by the anthraquinone process, anthraquinone (such as 2-ethylanthraquinone or the 2-amyl derivative) is reduced to the corresponding anthrahydroquinone, typically via hydrogenation on a palladium catalyst.
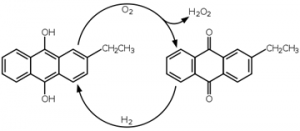 The anthrahydroquinone then undergoes to autoxidation to regenerate the starting anthraquinone, with hydrogen peroxide being produced as a by-product (1%). Hydrogen peroxide is then extracted.
The anthrahydroquinone then undergoes to autoxidation to regenerate the starting anthraquinone, with hydrogen peroxide being produced as a by-product (1%). Hydrogen peroxide is then extracted.
The anthraquinone derivative is reduced back to the dihydroxy (anthracene) compound using hydrogen gas in the presence of a metal catalyst. The cycle then repeats itself.
Chemical properties:
Acidic property:
Hydrogen peroxide is a week acid. In an aqueous solution it ionizes forming hydronium-ion and peroxide-ion:
H2O2 + H2O ⇄ H3O+ + O22–
Oxidation-reduction properties:
Hydrogen peroxide acts as both an oxidizing and a reducing agent.
In acidic solution it is an oxidizing agent:
2KI + H2O2 + H2SO4 → I2 + K2SO4 + 2H2O
However both in basic and in a neutral solutions hydrogen peroxide can be an oxidizing agent:
Cr2(SO4)3 + 3H2O2 + 10NaOH → 2Na2CrO4 + 3Na2SO4 + 8H2SO4
PbS + 4H2O2 → PbSO4 + 4H2O
At the presence of oxidizing agent it exhibits reduction properties in acidic, basic and neutral solutions:
Cl2 + H2O2 → 2HCl + O2;
Ag2O + H2O2 → H2O + O2 + 2Ag;
2KMnO4 + 5H2O2 + 3H2SO4 → 2MnSO4 + 5O2 + K2SO4 + 8H2O.
Decomposition of hydrogen peroxide:
Light, heating and the heavy metals hardly accelerate the process of hydrogen peroxide decomposition:
\(2{{H}_{2}}{{O}_{2}}\xrightarrow{Mn{{O}_{2}}}2{{H}_{2}}O+{{O}_{2}}\)
Uses:
- Its oxidizing properties are used in the bleaching of substances, such as hair, ivory, feathers, and delicate fabrics, which would be destroyed by other agents.
- It is used also medicinally, in the form of a 3% aqueous solution, as an antiseptic and throat wash.
- Hydrogen peroxide is used in restoring the original colors on paintings that have darkened through the conversion of the white lead used in the paintings to lead sulfide.
- The hydrogen peroxide oxidizes the black lead sulfide to white lead sulfate.
- It is used also as a source of oxygen in the fuel mixture for many rockets and torpedoes.
- It is used in synthesis of hydroquinone, tartaric acid and certain food products.
