Phase Change
1) Phase: We use the term phase to describe a specific state of matter, such as solid, liquid or gas. A transition from on phase to another is called a phase change.
i) For any given pressure a phase change takes place at a definite temperature, usually accompanied by absorption or emission of heat and a change of volume and density
ii) In phase change ice at \({{0}^{0}}C\) melts into water at \({{0}^{0}}C\). Water at \({{100}^{0}}C\) boils to form steam at \({{100}^{0}}C\)
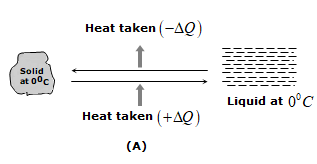
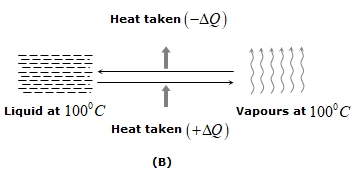
iii) In solids, the forces between the molecules are large and the molecules are almost fixed in their position inside the solid. In a liquid, the forces between the molecules are weaker and the molecules may move freely inside the volume of the liquid. However, they are not able to come out of the surface. In vapors or gases, the intermolecular forces are almost negligible and the molecules may move freely anywhere in the container.
When a solid melts, its molecules move apart against the strong molecular attraction. This needs energy which must be supplied from outside. Thus, the internal energy of a given body is larger in liquid phase than in solid phase. Similarly, the internal energy of a given body in vapor phase is larger than that in liquid phase.
iv) In case of change of state if the molecules come closer, energy is released and if the molecules move apart, energy is absorbed.
