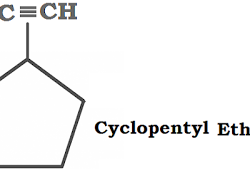
Qualitative Analysis of Organic Compounds
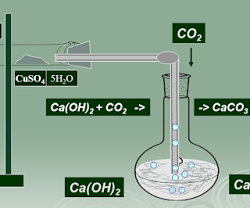
Qualitative chemical analysis deals with the identification of elements or grouping of elements present in a sample. Detection of Carbon and Hydrogen: Carbon and hydrogen are detected by heating the compound with copper (II) oxide. Carbon present in the compound is oxidised to carbon dioxide (tested with lime-water, which develops turbidity). C + 2CuO → 2Cu + CO₂ CO₂ Read more about Qualitative Analysis of Organic Compounds[…]