Developed by F. Hund and R.S. Mulliken in 1932
Features of this theory:
- The electrons in a molecule are present in the various Molecular orbitals.
- The atomic orbitals of comparable energies and proper symmetry combine to form molecular orbitals.
- In a molecular orbital an electron is influenced by two or more nuclei depending upon the number of atoms in the molecule.
- The number of molecular orbital formed is equal to the number of combining atomic orbitals.
- When two atomic orbitals combine, two molecular orbitals are formed. One is known as bonding molecular orbital while the other is called antibonding molecular orbital.
- The bonding molecular orbital has lower energy and hence greater stability than the corresponding antibonding molecular orbital.
- The molecular orbitals like atomic orbitals are filled in accordance with the Aufbau principle.
The formation of molecular orbitals may be described by the linear combination of atomic orbitals that can take place by addition and by subtraction of wave functions of individual atomic orbitals as shown below:
ψMO = ψA ψB
Therefore, the two molecular orbitals σ and σ* are formed as:
σ = ψA + ψB – bonding
σ* = ψA – ψB – Anti bonding
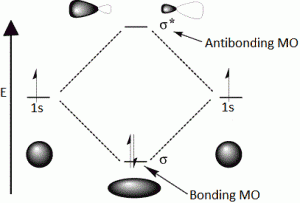
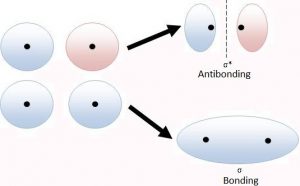
The electron density in a bonding molecular orbital is located between the nuclei of the bonded atoms because of which the repulsion between the nuclei is very less.
While in case of an antibonding molecular orbital, most of the electron density is located away from the space between the nuclei and hence the repulsion between the nuclei is high.
Conditions for the Combination of Atomic Orbitals:
- The combining atomic orbitals must have the same (or) nearly the same energy.
- The combining atomic orbitals must have the same symmetry about the molecular axis.
- The combining atomic orbitals must overlap the maximum extent.
Types of molecular bonds:
- Sigma bonds: Molecular orbitals that are symmetrical about the axis of the bond are called sigma molecular orbitals.
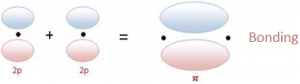
- Pi bonds: The π bonding is a side to side overlap of orbitals, which then causes there to be no electron density along the axis, but there is density above and below the axis.
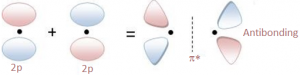
Energy level Diagram for Molecular Orbital:
- The sequence of energy levels of molecular orbitals for the total electron less than 14. σ1S < σ*1S < σ2S < σ*2S < (π2Px = π2Py) < σ2Pz < (π*2Px = π*2Py) < σ*2Pz.
- The important characteristic feature of this order is that the energy of σ2Pz molecular orbital is higher than that of π2Pz and π2Py.
- The sequence of energy levels of molecular orbitals for total electrons greater than 14 σ1S < σ*1S < σ2S < σ*2S < σ2Pz < (π2Px=π2Py) < (π*2Px=π*2Py) < σ*2Pz.
