Different Processes in Ideal Gas
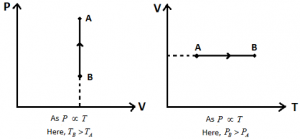
Isochoric Process: If the state of system changes in such a way that its volume remains constant, the process is called Isochoric Process.
Ideal Gas Equation: PV = nRT.
In case of Isochoric Process: V = Constant.
Hence, \(P=\left( \frac{nR}{V} \right)T\) (or) P α T.
Different graphs for Isochoric Process:
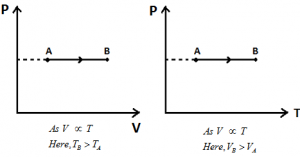
Isobaric Process: If the state of a system changes in such a way that pressure remains constant, the process is called Isobaric Process.
Ideal Gas Equation: PV = nRT (or) \(V=\left( \frac{nR}{P} \right)T\).
Here P = Constant. Hence, V α T.
Different graphs for Isobaric Process:
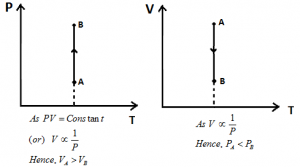
Isothermal Process: If the state of a system changes in such a way that temperature remains constant, the process is called as Isothermal Process.
Ideal Gas Equation: PV = nRT
Here, T = Constant
Hence, PV = Constant.
Different Graphs for Isothermal Process: