We already know that heat is a form of energy. It is a common observation that when we come out of the blanket on a cold morning we feel cold for some time but after that it becomes normal to some extent. So what exactly is happening? Initially heat begins from us to the surrounding and thus we feel cold. So till when heat will flow from us to surrounding? To answer this we introduce something known as temperature. It is basically the measure of hotness or coldness of a body.
Heat exchange takes place between two bodies till their temperatures become equal. Also it flows from body at higher temperature to one at lower temperature.
So, now we know about temperature we are in a position to define thermal equilibrium. It is defined as a situation in which no heat flows from one body to another when brought in contact with each another. As heat does not flow we can also say both body acquires same temperature at equilibrium.
One of the laws related to the thermal equilibrium is Zeroth Law of Thermodynamics.
Zeroth Law of Thermodynamics: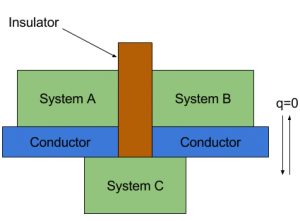 It states that by considering three systems A, B and C that initially are not in thermal equilibrium. We separate systems A and B with an adiabatic wall, but we let system C interact with both systems A and B. we wait until thermal equilibrium is reached, then
It states that by considering three systems A, B and C that initially are not in thermal equilibrium. We separate systems A and B with an adiabatic wall, but we let system C interact with both systems A and B. we wait until thermal equilibrium is reached, then
A and B are each in thermal equilibrium with C. but are they in thermal equilibrium with each other?
According to many experiments, there will be no net energy flow between A and B. This is an experimental evidence of the following statement.
If two systems are both in thermal equilibrium with a third body then they are in thermal equilibrium with each other.
This statement is known as the zeroth law of thermodynamics. It has this unusual name because it was not until after the great first and second laws of thermodynamics were worked out that scientists realized that this apparently obvious postulate needed to be stated first.
When zeroth law of thermodynamics means is that temperature is something worth measuring, because it indicates whether heat will move between objects. This will be true regardless of how the objects interact. Even if two objects don’t touch, heat may still flow between them, such as by radiation.
There are more formal ways to state the Zeroth Law of thermodynamics:-
Temperature is not mentioned clearly, but it’s implied that temperature exists. Temperature is the quantity that is always the same for all systems in thermal equilibrium with one another.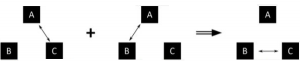 The double arrow represents the thermal equilibrium between systems. If systems A and C are in equilibrium, and systems A and B are in equilibrium, then systems B and C are in equilibrium. The systems A, B and C are at same temperature.
The double arrow represents the thermal equilibrium between systems. If systems A and C are in equilibrium, and systems A and B are in equilibrium, then systems B and C are in equilibrium. The systems A, B and C are at same temperature.
Why do you have a zeroth law of Thermodynamics: The Zeroth law defines thermal equilibrium. It also helps define the concept of temperature both of these are prerequisite assumptions and the concepts of first and second laws depends on.
Examples of Zeroth law of Thermodynamics: Consider 2 beakers of water in one beaker, the temperature of water is above the room temperature, and the other is below room temperature. They are left on a table, they are not in contact with each other, after some time, equilibrium reaches. Both beakers of ware are at the same temperature. The two beakers in thermal equilibrium with the surroundings, thus they are in thermal equilibrium with each other, and they are at the same temperature.
