Specific Heat of Liquid
Specific heat is the amount of heat in calories required to raise the temperature of 1 gram of a substance by 1 degree Celsius. The value depends on the liquid; water has a specific heat of 1 Cal/gram/degree Celsius. Ethyl alcohol has a specific heat of 0.58 Cal /degree Celsius. Mercury is 0.33, etc.
1) Among all known solids and liquids specific heat of water is maximum i.e. water takes more time to heat and more time to cool with respect to other solids and liquids.
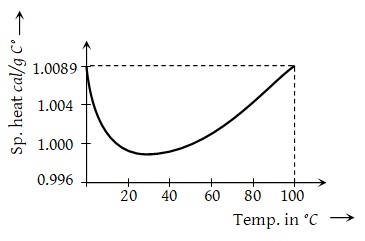
2) It is observed that by increasing temperature, initially specific heat of water goes on decreasing becomes minimum at \({{37}^{0}}C\) and then it start increasing.
Specific heat of water is:
\(\frac{1\,Cal}{gm{{\times }^{0}}C}=1000\frac{Cal}{kg{{\times }^{0}}C}=4200\frac{J}{kg{{\times }^{0}}C}\)This value is obtained between the temperatures \({{14.5}^{0}}C\) to\({{15.5}^{0}}C\).
3) The variation of specific heat with temperature for water is shown in the figure. Usually this temperature dependence of specific heat is neglected.
4) As specific heat of water is very large by absorbing or releasing large amount of heat its temperature changes by small amount. This is why; it is used in hot water or as coolant in radiators.
