We see so many changes happening around us every day, boiling of water, rusting of Iron, melting Ice, burning of paper etc. In all these processes we observe that the system in consideration goes from an initial state to a final state where some amount of heat is absorbed from the surrounding and some amount of work W is done by the system on the surrounding. Now, for how many of such systems can the system and the surrounding be brought back to their initial state? With common examples such as rusting and fermentation, we can say that in most of the cases it is not possible. In this section, we shall learn about the Reversible and Irreversible processes.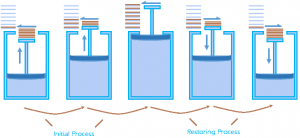 Reversible Processes: It is a process which can be made to proceed in the reverse direction by a very slight change in its conditions so that the system passes through the same states as in direct process, and at the conclusion of which the system and its surroundings acquire the initial conditions.
Reversible Processes: It is a process which can be made to proceed in the reverse direction by a very slight change in its conditions so that the system passes through the same states as in direct process, and at the conclusion of which the system and its surroundings acquire the initial conditions.
All Isothermal and adiabatic process when allowed to proceed slowly, are reversible, provided there is no loss of energy against any type of resistance.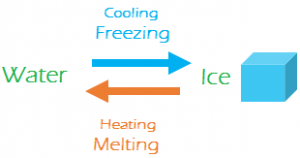 Examples of some Reversible Process:
Examples of some Reversible Process:
- One gram of ice at requires 80 Cal of heat to be converted into water at. Same amount of heat when taken out of water will convert it back to ice. So, fusion of ice is a reversible process.
- In terms of the reaction involving hydrogenation and dehydrogenation of milled LiH, the process seems to be reversible.
- One gram of water at gets converted into steam at by gaining 536 Cal of heat. Same amount of heat when taken out of steam converts it back to water. So, vaporization of water is also a reversible process.
- You can freeze orange juice to make an ice lolly and when you heat it the ice lolly become orange juice again. This is also an example of reversible process.
Basic Conditions to fulfil the process to be reversible:
- The change must take place at a very slow rate
- There should be no loss of energy due to the conduction or convection or dissipation of energy against any resistive effect like friction, viscosity etc.
- No heat should be converted into magnetic or electrical energy.
- The system must always be in thermal and mechanical equilibrium with the surrounding.
Irreversible Processes: An Irreversible process can be defined as a process in which the system and the surroundings do not return to their original condition once the process is initiated. Taking an example of an automobile engine, that has travelled a distance with the aid of a fuel equal to an amount ‘X’. During the process, the fuel burns to provide energy to the engine, converting itself into smoke and heat energy. We cannot retrieve back the energy lost by the fuel and cannot get back the original form. There are many factors due to which the irreversibility of a process occurs namely:
- The friction that converts the energy of the fuel to heat energy
- The unrestrained expansion of the fluid which prevents from regaining the original from of the fuel heat transfer through a finite temperature reverse of which is not possible as the forward process, in this case, is spontaneous
- Mixing of two different substances in nature, the reverse of which is not feasible.
The conditions mentioned above for the realisation of a reversible process are difficult to be met with, practically, since in every process some loss of energy is going to take place for one reason or the other. So most of the processes we come across in our daily life are Irreversible.
Thus some processes are reversible while others are irreversible in nature depending upon their ability to return to their original state from their final state.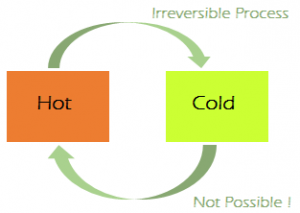 Examples of some Irreversible Process:
Examples of some Irreversible Process:
- Heat transfer through a finite temperature difference
- Flow of electric current through a resistance
- Unrestrained expansion of fluids
- Spontaneous chemical reactions
- Plastic deformation
