Quantitative analysis is the determination of the absolute or relative abundance (often expressed as a concentration) of one, several or all particular substance(s) present in a sample.
1. Carbon and Hydrogen:
Liebig’s combustion method: A known weight of organic compound is heated with pure and dry cupric oxide in a steam of pure and dry oxygen, when carbon is oxidized to carbon dioxide while hydrogen is oxidized to water. From the weight of CO₂ and H₂O, the percentage of C and H can be calculated.
CxHy + (x + y/4) O2 → x CO2 + (y/2) H2O
% carbon = weight of CO₂/ weight of organic compound x 12/44 x 100
% hydrogen = weight of H₂O/ weight of organic compound x 2/18 x 100
2. Nitrogen:
a) Duma’s method: The nitrogen containing organic compound, when heated with copper oxide in an atmosphere of carbon dioxide, yields free nitrogen in addition to carbon dioxide and water.
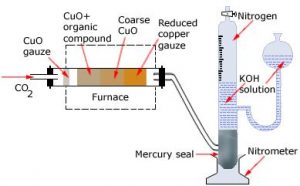
CxHyNz + (2x + y/2) CuO → x CO₂ + (y/2) H2O + z/2 N₂ + (2x + y/2) Cu
Traces of nitrogen oxides formed, if any, are reduced to nitrogen by passing the gaseous mixture over a heated copper gauze.
Oxides of nitrogen + Cu → N₂ + CuO
% of N = 28/22400 x V/W x 100
Where, V= volume of N₂ in ml at STP = p₁V₁ x 273/ 760 x T₁
Where p₁ and V₁ are the pressure and volume of nitrogen collected
p₁ = Atmospheric pressure – Aqueous tension
T₁ = room temperature
W = Weight of substance taken;
b) Kjeldahl’s method:
Includes 3 steps
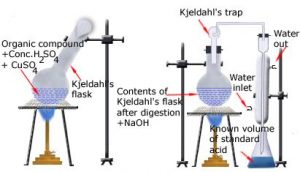
- The compound containing nitrogen is heated with concentrated sulphuric acid. Nitrogen gets converted to ammonium sulphate
Organic compound + H₂SO₄à (NH₄)₂SO₄ - The resulting acid mixture is then heated with excess of sodium hydroxide.
(NH₄)₂ SO₄ → NH₂ SO₄ + 2NH₃ + 2H₂O - The liberated ammonia gas is absorbed in an excess of standard solution of sulphuric acid. The amount of ammonia produced is determined by estimating the amount of sulphuric acid consumed in the reaction.
2NH₃ + H₂SO₄ à (NH₄)₂SO₄
It is done by estimating unreacted sulphuric acid left after the absorption of ammonia by titrating it with standard alkali solution. The difference between the initial amount of acid taken and that left after the reaction gives the amount of acid reacted with ammonia.
H₂SO₄ + 2 NaOH → Na₂SO₄ + 2H₂O
Percentage of N = 1.4 x M x 2(V – V₁/2)/m
M = Molarity of H₂SO₄
V (in mL) = Volume of H2SO4 of molarity, M
V1 (in mL) = Volume of NaOH of molarity, M, used for titration of excess of H2SO4
Note: This method is not applicable to compounds containing nitrogen in the ring (e.g. Pyridine, quinoline, etc.) and compounds containing nitro and azo (-N = N-) groups since nitrogen in these compounds is not completely converted into (NH₄)₂SO₄ during digestion
3. Halogens:
Carius method: The method is based on the fact that when an organic compound containing halogen (Cl, Br, or I) is heated in a sealed tube with fuming nitric acid in presence of silver nitrate, silver halide is formed. From the mass of silver halide formed, the percentage of the halogen can be calculated.
% of Cl = 35.3/ 143.5 x Mass of AgCl formed/Mass of Substance taken x 100
% of Br = 80/ 188 x Mass of AgBr formed/Mass of Substance taken x 100
% of I = 127/ 235 x Mass of AgI formed/Mass of Substance taken x 100
4. Sulphur:
Carius method: When an organic compound containing sulphur is heated with fuming nitric acid, sulphur is oxidised to sulphuric acid. This is precipitated as barium sulphate by adding barium chloride solution. From the amount of barium sulphate, percentage of sulphur can be calculated.
S + HNO₃ (fuming) → H₂SO₄
H₂SO₄ + BaCl₂ → BaSO₄ + 2HCl
White ppt
% of S = 32/233 x Mass of BaSO₄ formed/ Mass of substance taken x 100
5. Phosphorous:
Carius method: The organic compound containing phosphorus is heated with fuming nitric acid. Phosphorus is oxidised to phosphoric acid.
It is precipitated as magnesium ammonium phosphate, MgNH4PO4, by the addition of magnesia mixture (MgSO₄ + NH₄OH + NH₄Cl). The magnesium ammonium phosphate is washed, dried and ignited when it is converted to magnesium pyrophosphate Mg₂P₂O₇
2MgNH₄ PO₄ → Mg₂P₂O₇ + 2NH₃ + H₂O
From the mass of magnesium pyro-phosphate, the percentage of phosphorus in the compound can be calculated.
% of P = 62/222 = Mass of Mg₂P₂O₇ formed/ Mass of substance taken x 100
6. Oxygen:
- The usual method of determining the percentage of oxygen in an organic compound is by the method of difference. All the elements except oxygen present in the organic compound are estimated and the total of their percentages subtracted from 100 to get the percentage of oxygen
Percentage of oxygen = 100 – (Sum of the percentages of all other elements) - Aluise’s method: Organic compound containing oxygen is heated with graphite and CO formed is quantitatively converted into CO₂ on reaction with l₂O₅.
\(Org.compound\xrightarrow{Pyrolysis}Oxygen\)
\({{O}_{2}}+2C\xrightarrow{1100{}^\circ C}2CO\)
5CO + I₂O₅ → I₂ + 5 CO₂
% of O = 16/44 x Mass of CO₂ / Mass of Organic compound x 100
