It is the emission of electrons from the surface of certain substances, mainly metals, when they are illuminated by electromagnetic radiations like X-rays, ultraviolet and even visible light. The electrons emitted are called photo electrons.
Experiment to Demonstrate Photoelectric Effect:
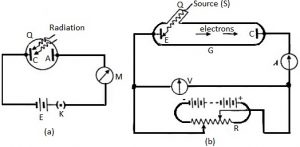
In figure (a) A and C are two zinc plates, connected to positive and negative of a battery E respectively, through a micro-ammeter M and a key K. The plates A and C are enclosed in an evacuated quartz bulb. The quartz will not absorb ultraviolet rays. It is evacuated, so that, the metal surface remains pure. The micro ammeter M registers a current, whenever ultraviolet rays fall on the zinc plate C. These electrons travel towards the plate A and thus a current flows through the circuit. If ultraviolet rays are stopped, there is no deflection in the micro ammeter. This means, electrons are ejected from the plate C by the radiations falling on it.
Laws of Photo Electric Emission:
- For a given photosensitive material, there is a minimum frequency below which there is no photoelectric emission. This frequency is independent of the intensity of light and is called the threshold frequency.
- Photoelectric emission is an instantaneous phenomenon. There is no time lag between the incidence of radiation and the emission of photo electrons.
- The number of photo electrons and the photoelectric current is directly proportional to the intensity of incident radiation (provided the frequency is greater than the threshold frequency).
- The kinetic energy of photo electrons increases with the frequency of the incident radiation and is independent of the intensity of radiation.
