Nuclear Fission
Nuclear Energy is the energy in the core of an atom. Where an atom is a tiny particle that constitutes every matter in the universe. Nuclear Energy is discharged by nuclear reactions either by fission or fusion. In nuclear fusion, atoms combine together to form a larger atom. In nuclear fission, the division of atoms takes place to form smaller atoms by releasing energy. Nuclear power plants produce energy using nuclear fission.
Nuclear Fission: When the nucleus of an atom splits into lighter nuclei through a nuclear reaction the process is termed as nuclear fission. This decay can be natural or can actually be simulated in a lab by achieving necessary conditions.
The resulting fragments tend to have a combined mass which is less than the original. The missing mass is what is converted into nuclear energy in the above reaction.
An example of nuclear fission is the splitting of Uranium – 235. The equation of the reaction has been given below.
²³⁵U₉₂ + ¹n₀ → ¹⁴⁴Ba₅₆ + ⁸⁹Kr₃₆ + 3 ¹n₀ + 210 Mev
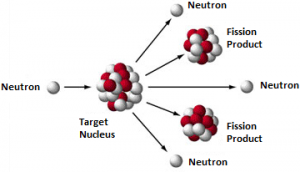
When a nucleus fission, it splits into several smaller fragments. These fragments or fission products are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This “missing” mass has been converted into energy according to Einstein’s equation: Fission can occur when a nucleus of a heavy atom captures a neutron or it can happen spontaneously.
