Heat Engine Efficiency
A heat engine is a device that converts heat into work continuously through a cyclic process. It takes heat from a reservoir then does some like moving a piston, lifting weight etc. and finally discharging some of the heat energy into the sink. Schematically it can be represented as:
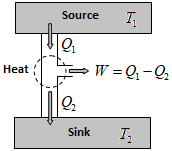
The essential parts of a heat engine are:
1) Source: It is a reservoir of heat at high temperature and infinite thermal capacity. Any amount of heat can be extracted from it.
2) Working Substance: Steam, Petrol etc.
3) Sink: It is a reservoir of heat at low temperature and infinite thermal capacity. Any amount of heat can be given to the sink.
The working substance absorbs heat Q₁, from the source, does an amount of work W, return the remaining amount of heat to the sink and comes back to its original state and there occurs no change in its internal energy. By repeating the same cycle over and over again, work is continuously obtained.
The performance of heat engine is expressed by mean of efficiency (η), which is defined as the ratio of useful work obtained from the engine to the heat supplied to it.
\(\eta =\frac{Work\,\,done}{Heat\,\,input}=\frac{W}{{{Q}_{1}}}\),
For cyclic process, ΔU = 0 hence, ΔQ = ΔW; So, W = Q₁ – Q₂ ⇒ \(\eta =\frac{{{Q}_{1}}-{{Q}_{2}}}{{{Q}_{1}}}=1-\frac{{{Q}_{2}}}{{{Q}_{1}}}\),
A perfect heat engine is one which converts all heat into work. d i.e.., W = Q₁ so that Q₂ = 0 and hence η = 1. But practically efficiency of an engine is always less than 1.
