We have learnt about heat, internal energy and work. We also know how these are related to states of the system. But before going any further we need to define thermodynamics. It is a process that brings about change in the state of a system. Now which quantities determine state of system? These are pressure, volume, temperature, mass, internal energy etc. these quantities are referred as state variable and measured only at equilibrium. Why equilibrium? Suppose I heated a container containing gas from one end, initially the temperature will not be same everywhere but finally it will reach an equilibrium value. So we will consider that value. We can classify state variables further as intensive or extensive. Intensive variables are independent of the dimension of the systems like pressure and temperature, while extensive variables depend on dimensions of the system like volume, mass internal energy etc.
First Law of Thermodynamics: It is a conservation of energy. The law states that the total energy of an isolated system is constant, energy can be transformed from one form to another form, but can’t be created or destroyed. This can also be stated as the energy of a system has to be equal to the work that is being done, plus or minus the heat that flow in or out of the system.
And this can also be states that this law relates heat, internal energy and work. According to this law, some of the heat given to system is used to change the internal energy while the rest is used in doing work by the system. It can be represented mathematically as,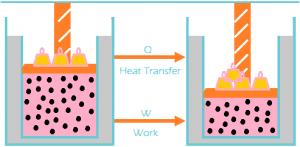 ΔU = ΔQ – W
ΔU = ΔQ – W
Where,
ΔQ = Heat given or lost
ΔU = Change in internal energy
W = Work Done
So we can infer from the above equation that the quantity (ΔQ – W) is independent of the path taken to change the state. Further we can say that internal energy tends to increase when heat is given to the system and vice – versa.
Any thermodynamic system in an equilibrium stat possesses a state variable called the internal energy. Between any two equilibrium states, the change in internal is equal to the difference of the heat transfer into the system and work done by the system.
How to find the First Law of Thermodynamics:
Example: Find the internal energy change and temperature change for the two processes involvint one mole of an ideal monoatomi gas.
a) 1500 J of heat are added to the gas and the gas does no work and no work is done on the gas?
b) 1500 J of work are done on the gas and the gas does no work and no heat is added or taken away from the gas?
Solution:
a) ΔU = Q – W
⇒ ΔU = 1500 – 0 = 1500 J
⇒ ΔU = 1500 J
⇒ \(\Delta U\,=\,\left( \frac{3}{2} \right)\,n\,R\,\Delta T\)
⇒ \(1500\,=\,\,\left( \frac{3}{2} \right)\,\times 1\times 8.31\times \Delta T\)
ΔT = 120 K
b) ΔU = Q – W
⇒ ΔU = 0 – 1500 = 1500 J
⇒ ΔU = 1500 J
⇒ \(\Delta U\,=\,\left( \frac{3}{2} \right)\,n\,R\,\Delta T\)
⇒ \(1500\,=\,\,\left( \frac{3}{2} \right)\,\times 1\times 8.31\times \Delta T\)
ΔT = 120 K
In both the processes, the change in internal energy is the same. We say that the internal energy is a state function. A state function depends only on the state of the system and not on the process that brings the system to that particular state.
