Electric Dipole
An electric dipole is a pair of two objects having equal and opposite charges, separated by a distance d. suppose there are two charges of equal magnitude, separated by a distance d. Assume the first charge be negative and the second charge be positive, this combination is called as an Electric dipole. Therefore, an electric dipole is created by the combination of equal and opposite charges by a separation of a certain distance.
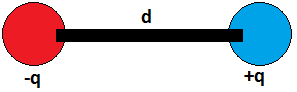
Electric dipole is denoted by \(\overrightarrow{p}\) and equals to product of charge and distance \(\overrightarrow{d}\) between them.
Electric dipole \(\left( \overrightarrow{p} \right)\,\,=\,\,q\overrightarrow{d}\).
Electric dipole moment is a vector quantity, it has a defined direction which is from the negative charge to the positive charge.
Electric dipoles are common in nature, sot the analysis of them has many practical applications:
Dipoles are usually found in molecular structures caused by non – uniform charge distribution of protons and electrons, and are used to find the polarity of a system which is useful in understanding many chemical phenomena such as surface tension, melting/ boiling points and solubility.
How to find the Electric dipole moment?
Problem: What is the dipole moment for a dipole having equal charges -2C and +2C separated with a distance of 2cm?
Solution: Given,
Distance (d) = 2 cm = 0.02m
Charge (q) = 2C
We know that:
Diploe moment (p) = q x d = 2 x 0.02 = 0.04 cm
Therefore,
Dipole moment (p) = 0.04 cm.
