Degrees of Freedom
The degrees of freedom can be calculated to help ensure the statistical validity of t – tests and even the more advanced f – tests. The number of degrees of freedom refers to the difference between the numbers of independent observations in a sample and the number of population parameters that must be estimated from sample data.
What is Degrees of Freedom?
The number of degrees of freedom of a dynamical system is defined as the total number of co – ordinates or independent variables required to describe the position and configuration of the system. If a particle is moving a straight line along any one of the axis, it has one degree of freedom. If a particle is moving in a plane, it has two degrees of freedom. If a particle is moving in space, it has three degrees of freedom.
A point mass cannot undergo rotation, but only translatory motion. A rigid body with finite mass has both rotatory and translatory motion. The rotatory motion also can have three co-ordinates in space, like translatory motion. Therefore, a rigid body will have six degrees of freedom. Three dues to translatory motion and three dues to rotatory motion.
Monoatomic Molecule: Since a monoatomic molecule consists of only a single atom of point mass it has three degrees of freedom of translatory motion along the three co-ordinate axes as shown in the below figure.
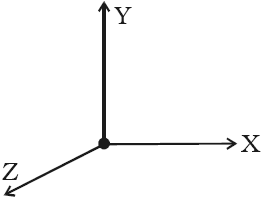
Example: Molecules of rare gases like helium, argon, etc.
Diatomic Molecule: The diatomic molecule can rotate about any axis at right angles to its own axis. Hence it has two degrees of freedom of rotational motion in addition to three degrees of freedom of translational motion along the three axes. So, a diatomic molecule has five degrees of freedom as shown in the below figure.
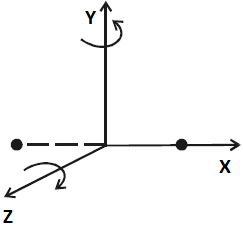
Examples: Molecules of N₂, CO, Cl₂, etc.
Triatomic Molecule: In the case of triatomic molecule of linear type, the centre of mass lies at the central atom. It, therefore, behaves like a diatomic molecule with three degrees of freedom of translation and two degrees of freedom of rotation, totally it has five degrees of freedom as shown in below figure.
![]()
Examples: Molecules of CO₂, CS₂, etc.
