Charles’s Law
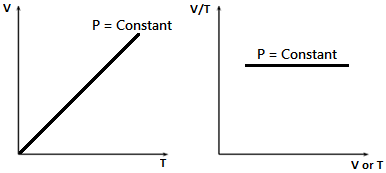
Charles’s law states that when keeping the pressure constant, the volume of a gas varies directly with the temperature. Charles’s law equation can be represented as V α T.
Where,
V = Volume of the gas,
T = Temperature.
This law dictates the linear relationship that volume shares with temperature. The temperatures are conventionally measured in Kelvin, the SI unit of temperature. At a given pressure, the volume of a given mass of a gas is proportional to its absolute temperature. This is known as Charles’s law.
From Kinetic theory of gases, if P is constant, V α v²rms.
As v²rms α T, we get V α T which is Charles’s law.
V/ T = Constant
V₁/ T₁ = V₂/ T₂
V/ T = m/ ρT = Constant (As Volume (V) = m/ ρ)
ρT = Constant
ρ₁T₁ = ρ₂T₂.
Thus, V – T graph is a straight line passing through origin (or) V/T versus V or T graph is a straight line parallel to V or T axis.