Boyle’s Law
Robert Boule studied the variation of volume of a given mass of gas with pressure at constant temperature. The result of his study in the form of law which is known as Boyle’s Law. Boyle’ Law states that the volume of a given mass of a gas is inversely proportional to its pressure at constant temperature. From Kinetic theory of gases, we have:
PV = ⅓ mNv²rms … (1)
As for a given gas v²rms α T the value of v²rms is constant at a given temperature also for a given mass of the gas m and N are constants. Thus, from equation (1)
PV = Constant
P α 1/ V, which is Boyle’s law.
Also, we can write it as:
PV = P (m/ ρ) = Constant
Where,
m = Mass of the gas,
ρ = Density of gas,
P/ ρ = Constant.
\(\frac{{{P}_{1}}}{{{\rho }_{1}}}=\frac{{{P}_{2}}}{{{\rho }_{2}}}\).
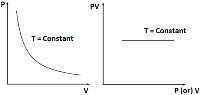
Thus, P – V graph is a rectangular hyperbola or PV versus P or V graph is a straight line parallel to P or V axis.