Application of first law of Thermodynamics on different process we have the different process like.
- Cyclic process
- Isolated process
- Isothermal process
- Isobaric process
- Isochoric process
- Adiabatic process
Cyclic process:-
When a system is taken form an initial state to other state and finally brought back to the initial state, then it is called “Cyclic Process”.
We know that internal energy is a function of state of system
So there will be no change in the internal energy
=> ΔV = 0
We know that
ΔV = Q – W
=>Q – W = 0
Q = W
So we can conclude that for a cyclic process total heat energy given will be converted to work.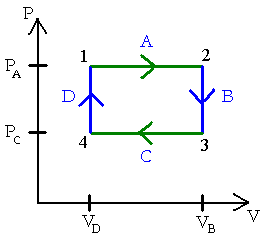 Isolated system:-
Isolated system:-
A system which can neither do work nor can take or give heat from or to the outside is called an isolate system for such process Q = W = 0.
ΔV = Q – W
=>ΔV = 0; change in internal energy = 0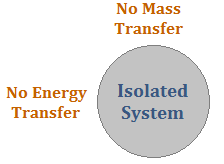 Isochoric process:–
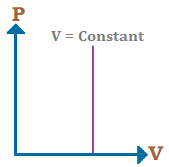
Isochoric process:–
The process which takes place at constant volume is called isochoric process.
For an isochoric process ΔV = 0
We know that
W = pΔV = P (0) = 0
W = 0
ΔV = Q – W
ΔV = Q
So for isochoric process heat energy is completely converted to change in internal energy.