Alkynes:
- These are unsaturated hydrocarbons containing at least one triple bond between two carbons.
- General formula is Cn H2n-2, (n = 2, 3, 4, 5, …).
- Acetylene is first member
Nomenclature and isomerism:
The IUPAC name is derived from the IUPAC name of alkanes by replacing ending ‘ane’ by ‘yne’ along with the position of triple bond e.g. Alkane–ane + yne = Alkyne.
Ex: CH3 – CH2C ≡ CH but – 1 – yne
|
Value of n |
Formula | Structure | Common name | IUPAC name |
| 2 | C₂H₂ | HC ≡ CH | Acetylene |
Ethyne |
| 3 | C₃H₄ | CH₃ – C ≡ CH | Methyl Acetylene |
Propyne |
Alkynyl groups:
Residual part left after the removal of one H atom from alkyne is known as alkynyl group. According to IUPAC nomenclature, these groups are named by replacing terminal (e) of alkyne by “yl” e.g.
|
HC ≡ C – |
ethynyl |
| HC ≡ C – CH2 – |
2-propynyl |
|
– C ≡ C – CH3 |
1-propynyl |
Structure of acetylene:
- Acetylene contains one ‘sigma’ bond and two ‘pi’ bonds.
- Sigma bond is formed by the head on overlapping of the two sp hybridized orbitals of the two carbon atoms.
- The remaining sp hybridized orbital of each carbon atom undergoes overlapping along the inter-nucleus axis with 1s orbital of the two hydrogen atoms forming two C – H bonds. H – C – C bond angle is 180⁰C.
- The unhybridised orbitals are perpendicular to the plane of C – C sigma bond these are on sidewise overlapping gives two ‘pi’ bonds.
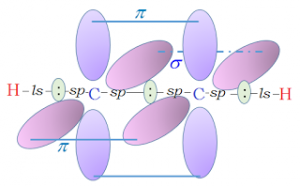
- Carbon–carbon bond strength is more than that of alkenes and alkanes and acetylene molecule is linear structure.
Isomerism:
- Isomerism in alkenes starts from four carbons.
CH₃ – CH₂ – C ≡ CH – 1 – butyne
CH₃ – C ≡ C – CH₃ – 2 – Butyne - These are positional isomers due to different positions of triple bond.
