Adiabatic Process
Adiabatic process is a thermodynamic process in which there is no heat (Q = 0) transfer into or out of a system and is generally obtained by surrounding the entire system with a strongly insulating material or by carrying out the process so quickly that there is no time for a significant heat transfer to take place.
Applying the first law of thermodynamics to an adiabatic process, we obtain:
ΔU = -W
Where,
ΔU = Change in Internal Energy
W = Work done by the system
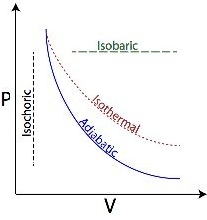
In adiabatic process the working substance is perfectly insulated from the surroundings. All along the process there is change in temperature.
When work is done on the working substance, there is rise in temperature, because, the external work done on the working substance increases the internal energy. When work is done by the working substance, it is done on the cost of its internal energy, that’s why, there is fall in temperature.
The equation for an adiabatic process is PVγ = Constant
Examples: There are several instances, some are stated below:
- It is a process where there is a gas compression and heat is generated. One of the simplest examples would be release of air from a pneumatic tire.
- Adiabatic efficiency is applied to devices such as nozzles, compressors and turbines. One of the good applications of the adiabatic process.
- The pendulum oscillating in a vertical plane is an example of it.
- A quantum harmonic oscillator is also an example of adiabatic system.
- When we put the ice into the ice box, no heat goes out and no heat comes in.
