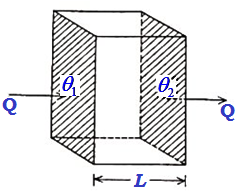
One-dimensional Flow of Heat: Coefficient of Thermal Conductivity
Let us consider a parallel-faced slab whose cross-sectional area is A and length L. Let its faces be maintained at steady temperatures θ1 and θ2. Heat will flow through the slab from the face at higher temperature θ1 to that at the lower temperature θ2. Let us assume that no heat escapes out from the Read more about One-dimensional Flow of Heat: Coefficient of Thermal Conductivity[…]