1. Effect of temperature on reaction rate:
⇒ As the temperature increases, the average energy of the reaction increases, hence rate of reaction increases.
⇒ As a common rule, for every 10° rise of temperature, the rate of reaction will increase by 2 (or) 3 times of initial value.
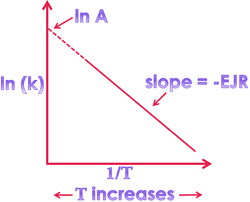
⇒ According to Arrhenius concept:
η = η₀ e -Eₐ/RT
η₀ = total number of molecules
η = total number of molecules activated
Eₐ = Activation energy
It can be written in the following way.
K = A e -Eₐ/RT
K = Rate Constant of reaction
log K₂/K₁ = -Eₐ/R ([1/T₂] – [1/T₁])
2. Effect of catalyst on reaction rate:
⇒ A catalyst alters the reaction velocity by changing the activation energy by changing the reaction mechanism, without getting consumed during the reaction.
⇒ The physical state of the catalyst may or may not change.
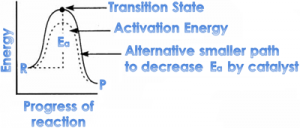
⇒ A catalyst works by providing an alternate path to the reaction
a. Positive catalysts: A catalyst which increases the rate of reaction is called positive catalyst.
Such catalyst decreases activation energy by accepting a smaller path, so rate of reaction is increased.
Eg: \(2S{{O}_{2}}\left( g \right)+{{O}_{2}}\left( g \right)\xrightarrow{Pt\left( s \right)}2S{{O}_{3}}\left( g \right)\)
b. Negative catalysts (Inhibitors): A catalyst which decreases or retards the rate of reaction is called negative catalysts.

It is because a -ve catalyst increase activation energy by taking a longer alternative path.
Eg: \(2{{H}_{2}}{{O}_{2}}+\xrightarrow[or\,\,Glycerine]{{{H}_{3}}P{{O}_{4}}\left( s \right)}2{{H}_{2}}O+{{O}_{2}}\)
