Physical properties:
- Physical properties are smaller to alkenes and alkanes.
- First three are gases, next eight are liquids and higher ones are solids.
- These are insoluble in water and soluble in organic solvents.
Chemical properties:
a) Acidic character of acetylene: Acetylene reacts with sodamide to form sodium acetylide with liberation of hydrogen gas.
HC ≡ CH + Na → HC ≡ C⁻ Na⁺ + ½ H₂
Mono sodium ethynide
The acidic character of ethyne compared to ethene and ethane is due to the greater percentage of S-Character.
In acetylene the percentage of s-character is 50% when as in case of ethylene and ethane are 33.3% and 25% respectively.
Only hydrogen atoms attached to triply bonded carbon atoms are acidic in nature.
CH₃ – C ≡ CH + Na⁺ NH₂⁻ → CH₃ – C ≡ C⁻ Na⁺ + NH₃
b) Addition of reagent: Due to presence of triple bond alkynes undergo addition reactions with hydrogen and halogens, hydrogen halides.
c) Addition of dihydrogen:
\(HC\equiv CH+{{H}_{2}}\xrightarrow{Pt/Pd/Ni}\left[ {{H}_{2}}C=C{{H}_{2}} \right]\xrightarrow{{{H}_{2}}}C{{H}_{3}}-C{{H}_{3}}\)d) Addition of halogens:
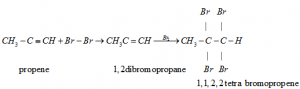
e) Addition of hydrogen Halides: Two molecules of hydrogen halides added to alkynes to form gem dihalides
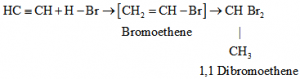
f) Addition of water: Alkynes add to water molecule in presence of mercuric sulphate and dilute sulphuric acid at 333K to form carbonyl compounds.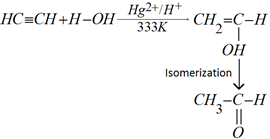
g) Polymerization:
- Linear Polymerization: Under suitable conditions linear polymerization of etheyne gives polyethylene.
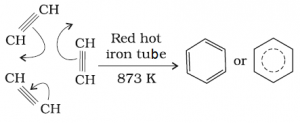
[CH = CH – CH = CH] is repeating until [- CH = CH – CH = CH -)n - Cyclic polymerization: Acetylene passing through a red hot iron tube at undergoes cyclic polymerization. Three molecules polymerise to form benzene.