Photo Electric Effect
Photoelectric effect or photoelectric emission is a phenomenon where electrons are ejected from the surface of a metal upon shining light on it. Energy which is contained within the light of incidence is getting absorbed by electron particles within the metal which escape the metallic surface given enough energy. The photoelectric effect was first observed by Heinrich hertz in 1887.
The laboratory setup typically involves a photodiode. The cathode is clean surface of a metal is exposed to light and is expected to yield electrons. The surface used to collect electrons is the anode which faces the cathode, and both are sealed in vacuum.
Light of different intensities and frequencies (colours) is shone onto the cathode that emits electrons that are collected on the anode. The stream of electrons forms from cathode to anode is called photoelectric current.
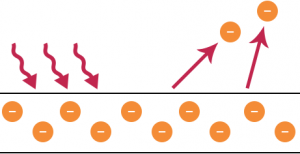
Experimental Result of Photoelectric Emission: By utilizing the Maxwell’s wave theory of light, it is understood that the more intensive the incident light is the greater the energy with which the electrons get ejected from the metal. This implies that the average energy carried by an emitted electron should increase with the continuous increase in the intensity of the incident light. However this is not the case observed by Lenard, an assistant of Hertz who performed the first conclusive study of photoelectric emission of electrons.
The results were startling and challenged the foundation of classical thinking in physics. Lenard’s experiment suggested that the intensity (measure of energy) of the incident light had no effect on the maximum kinetic energy of the photoelectrons which is rather dependent on the frequency (or wavelength) of the incident light.
Those emitted as a response to a bright light had the same energy as those emitted from exposure to a dim light of the same frequency. However, the photoelectric current increases with higher intensity of light. This occurs not because of individual electrons gaining more kinetic energy, but as the number of ejected photoelectrons goes up with brighter incident light, which is in accordance with the law of conservation of energy.
Later experiments showed that light above a certain wavelength, corresponding to frequencies below a certain cut off value, called the threshold frequency, would not result in ejection of photoelectrons from the surface of a metal irrespective of the intensity of the incident light.
Einstein’s Photoelectric Effect: Classical electromagnetic wave theory of light fails to account for this behaviour which was later correctly explained by Einstein in 1905.
Einstein successfully helped understand this by suggesting that the light of incidence was composed of photons or basically the individual quanta which interacted with the metal surface electrons as discrete particles instead of a wave like continuum.
For a given frequency (or wavelength) of the incident light, each photon carried the energy,
E = hf
Where h is considered to be the Planck’s constant while f as frequency.
By increasing the light intensity there was an increase in the flux of photoelectrons, i.e. the number of incident photons per unit times with the energy of each photon staying the same when the frequency (or wavelength) of the incident light is fixed. In other words, photoelectric effect established the corpuscular nature of light as opposed to the 19th century notion of light as a continuous spectrum of electromagnetic wave.
Einstein’s equations for the photoelectric effect: According to the Einstein, the photoelectric effect is,
Energy of photon = Energy needed to remove an electron + kinetic energy of the emitted electron
hv = W + E
Where,
h – Planck’s Constant.
v – Frequency of the Incident Photon.
W – Work Function.
E – The maximum kinetic energy of ejected electrons.
Applications of Photo Electric Effect:
- Photocells and solar cells rely on photoelectric effect to generate electricity. Solar cells harness the radiated energy of the Sun on Earth. Solar power is widely used as a cost-effective and clean alternative source of energy.
- As long as the beam of light strikes the photocell, the photoelectric effect produces enough ejected electrons to give rise to a detectable electric current.
- By blocking and allowing light to the photocell, it can be used to automate doors, alarms and has a broad application in security systems. Other uses include television camera tubes and light-activated counters.
