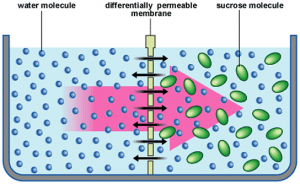
Osmosis:
⇒ The movement of solvent molecules through a semi-permeable membrane (kept between a pure solvent and its solution) from pure solvent to solution is osmosis.
⇒ The movement of solvent molecules continues till equilibrium is reached.
⇒ Solvent molecules move from lower concentration solution to higher concentration solution.
Osmotic pressure:
⇒ The excess pressure exerted on the solution side to stop the movement of solvent molecules into the solution.
⇒ Depends on concentration of solution
At a given temperature,
π α C
π = CRT
π = Osmotic Pressure
C = molarity
R = gas constant
T = Temperature
Isotonic solutions: Two solutions having same osmotic pressure at a given temperature.
Hypertonic solution: Any solution with a higher salt concentration than normal body cells so that the water is drawn out of the cells by osmosis. Hypertonic solutions have higher osmotic pressure.
Hypotonic solution: any solution with a lower concentration than normal body cells so that water flows into the cells by osmosis. Hypotonic solutions have lower osmotic pressure.
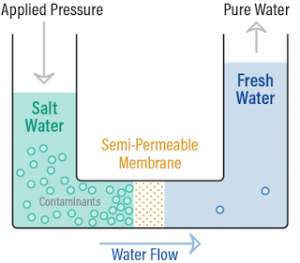
Reverse Osmosis:
⇒ When pressure greater than osmotic pressure is applied on the solution, the solvent molecules move from solution to pure solvent. This is reverse osmosis
⇒ Used in sea water purification
⇒ Reverse osmotic pressure > osmotic pressure.
Determination of molar mass: We can determine the molecular weights of the solute using colligative properties.
The molecular weight Relates with magnitude of a colligative properties as.
Molecular Mass α 1/ Magnitude of colligative property
1. From vapour pressure:
\(\frac{\Delta {{P}_{A}}}{{{P}_{A}}}=\frac{{{w}_{B}}\times {{M}_{A}}}{{{M}_{B}}\times {{w}_{A}}}\) \({{M}_{B}}=\frac{{{P}_{A}}\times {{w}_{B}}\times {{M}_{A}}}{\Delta {{P}_{A}}\times {{w}_{A}}}\)Where, wA = mass of solvent
MA = Molar mass of solvent
WB = mass of solute
MB = Molar mass of solute
2. From boiling point:
\({{M}_{2}}=\frac{1000\times {{w}_{2}}\times {{K}_{b}}}{\Delta {{T}_{b}}\times {{w}_{1}}}\)M2 = molar mass of solute
W2= mass of solute
Kb = Molal elevation constant
∆Tb = elevation in boiling point
3. From freezing point:
\({{M}_{2}}=\frac{1000\times {{w}_{2}}\times {{K}_{f}}}{\Delta {{T}_{f}}\times {{w}_{1}}}\)M2 = molar mass of solute
W2= mass of solute
Kf = Molal depression constant
∆Tf = depression in freezing point
4. From osmotic pressure:
\({{M}_{2}}=\frac{{{w}_{2}}\times R\times T}{\pi \times V}\)M2 = molar mass of solute
R = gas constant
T = temperature
π = osmotic pressure
V = Volume of the solution in liters.
