According to the law of equipartition of energy, for any dynamic system in thermal equilibrium, the total energy for the system is equally divided among the degrees of freedom.
The kinetic energy of a single molecule along the x- axis, y- axis and z- axis is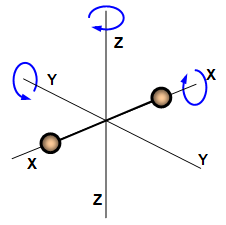 Kinetic energy of single molecule is Et = ½ m v2x + ½ m v2y + ½ m v2z
Kinetic energy of single molecule is Et = ½ m v2x + ½ m v2y + ½ m v2z
|Et| = 3/2 KBT
Let us take
|½ m v2x |= ½ KBT
|½ m v2y |= ½ KBT
|½ m v2z |= ½ KBT
Motion of a body from one point to another is called translation.
The number of independent ways. By which a molecule can move, without violating any constraint imposed unit, is called number of degrees of freedom.
Molecules of a monatomic gates have only translational degrees of freedom. Diatomic gas has both translational degrees of freedom as well as rotational degrees of freedom around their centre of mass
So.
E = Et + Er = ½ m v2x + ½ m v2y + ½ m v2z + ½ I1 ω21 + ½ I1 ω22
Et = translational energy
Er = rotational energy
I1 & I2 are moment of interior ω1 & ω2 are angular velocities.
Some molecules even have vibrational energy [Ev] also.
So, E = ET + ER + EV
EV can written as Ev = ½ m (dy/dt)² + ½ KY²
Where K = constant
Y = vibrational coordinate [Y]
In equilibrium, the total energy is equally is tribute in all possible energy modes with each mode having an average energy equal to ½ KBT this is known as “law of equipartition of energy”.
