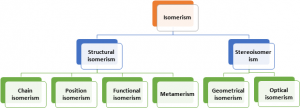
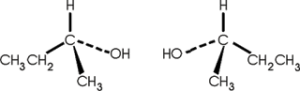Isomerism
Isomerism is a phenomenon shown by two or more organic compounds having the same molecular formula but different properties due to difference in arrangement of atoms along the carbon skeleton (structural isomerism) or in space (Stereo isomerism)
Structural isomerism
1. Chain isomerism: These isomers arise because of the possibility of branching in carbon chains. For example, there are two isomers of butane, C4H10. In one of them, the carbon atoms lie in a “straight chain” whereas in the other the chain is branched.
\(\underset{bu\tan e({{C}_{4}}{{H}_{10}})}{\mathop{\underset{{}}{\mathop{H-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-H}}\,}}\,\,\,\,\,\underset{2-methylpropane({{C}_{4}}{{H}_{10}})}{\mathop{\underset{{}}{\mathop{H-\overset{H}{\mathop{\overset{|}{\mathop{\overset{H}{\mathop{\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,}}\,-\underset{H}{\overset{-\,\,\,\,\,\,C\,\,\,\,\,-}{\mathop{\overset{|}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}\,}}}\,-\overset{H}{\mathop{\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,}}\,}}\,}}\,-H}}\,}}\,\)2. Positional isomers: The isomersthat have the same carbon skeleton and the same functional groups but differ from each other in the location of the functional groups on the carbon chain.
\(\underset{1-\,Chloropen\tan e}{\mathop{H-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-Cl}}\,\,\,\,\,\,\,\underset{2-Chloropen\tan e}{\mathop{H-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{Cl}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-H}}\,\)3. Functional group isomerism: The isomers contain different functional groups – that is, they belong to different families of compounds (different homologous series).
Ex: \(\underset{dimethyl\,\,ether\,({{C}_{2}}{{H}_{6}}O)}{\mathop{H-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-O-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-H}}\,\,\,\,\underset{ethanol\,({{C}_{2}}{{H}_{6}}O)}{\mathop{H-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-O-H}}\,\)
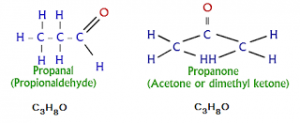
4. Metamerism: Themetamerismarises when different alkyl groups are attached to same functional group.
Eg: \(\underset{Diethyl\,\,ketone\,\,(pen\tan -3-one)}{\mathop{{{H}_{5}}{{C}_{2}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-{{C}_{2}}{{H}_{5}}}}\,\,\,\,\underset{Methyl\,\,propyl\,\,ketone\,\,(pen\tan -2-one)}{\mathop{{{H}_{3}}C-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-{{C}_{3}}{{H}_{7}}}}\,\)

Stereoisomerism
The isomerism which arises due to different spatial arrangements of atoms or groups is called stereo isomerism. It is broadly divided into:
- Geometrical isomerism
- Optical isomerism
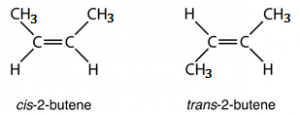
1. Geometrical isomerism: The geometrical isomerism arises when atoms or groups are arranged differently in space due to restricted rotation of a bond or bonds in a molecule.
2. Optical isomerism: Involves an atom, usually carbon, bonded to four different atoms or groups of atoms. They exist in pairs, in which one isomer is the mirror image of the other.
e.g. butan-2-ol