Isochoric Process
An Isochoric process is a thermodynamic process in which the volume remains constant. Since the volume is constant, the system does no work (W = 0). It is also called as constant volume process, and Iso – Volumetric process or an isometric process is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. The Isochoric process here should be a Quasi – Static Process.
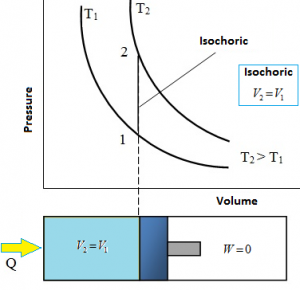
Relation between P, V and T:
P/T = Constant
Work done by the gas:
W₁₋₂ = P (V₂ – V₁) = 0
Change in Internal Energy:
dU = mCV (T₂ – T₁)
U₂ – U₁ = mCV (T₂ – T₁)
Heat supplied or Heat Transfer:
Q₁₋₂ = dU + W₁₋₂
Q₁₋₂ = U₂ – U₁ = mCp (T₂ – T₁)
Change in Enthalpy:
H = mCp (T₂ – T₁)
Important points:
- The change in internal energy (dU) and the change in enthalpy (dH) have the same expression for each process.
- During expansion or heating process, work is done by the gas (i.e. W₁₋₂ is +ve); internal energy of the gas decreases (i.e. dU is -ve) and the heat supplied to the gas (i.e. Q₁₋₂ is +ve).
- During compression or cooling process, work is done on the gas (i.e. W₁₋₂ is -ve); internal energy of the gas increases (i.e. dU is +ve) and the heat supplied by the gas (i.e. Q₁₋₂ is -ve).
