- When sigma electrons are in conjugation with pi- bond then this conjugation is known to be ‘Hyper conjugation’.
- Generally used to compare stability.
Required Conditions:
- Compound should have at least one sp2 hybridized carbon atom of alkene or alkyl carbocation or alkyl free radical.
- Alpha carbon with respect to sp2 hybrid carbon must be sp3 and it should have at least one alpha-Hydrogen.
If both the conditions are fulfilled then only “Hyper conjugation” occurs otherwise not.
Types of Hyper conjugation:
- π conjugation
- Positive charge conjugation
- Free radical conjugation
Ex 1: Let’s consider an example of CH₃ – CH = CH₂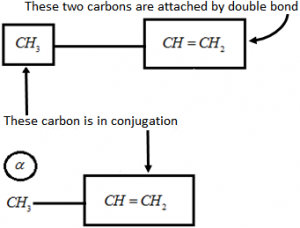 Alpha-carbon is the carbon attached to the sp2 – hybrid carbon.
Alpha-carbon is the carbon attached to the sp2 – hybrid carbon.
Alpha-Hydrogen is the Hydrogen attached to the alpha-carbon atom.
Hence, \(\alpha \,\,H-\underset{\alpha \,\,\,H}{\overset{\alpha \,\,\,H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\)
This compound has three alpha-hydrogens
Type 1: Pi-Conjugation
- \(C{{H}_{3}}-\overset{\alpha }{\mathop{C{{H}_{2}}}}\,-CH=C{{H}_{2}}\)
Two alpha hydrogens in the above compound.
- \(\overset{\alpha }{\mathop{C{{H}_{3}}}}\,-CH=CH-\overset{\alpha }{\mathop{C{{H}_{2}}}}\,-C{{H}_{3}}\)
Type 2 : Positive Charge conjugation
- \(\overset{\alpha }{\mathop{C{{H}_{3}}}}\,-\overset{\oplus }{\mathop{C{{H}_{3}}}}\,\)
Three alpha-hydrogens in the above compound.
- \(\overset{\alpha }{\mathop{C{{H}_{3}}}}\,-\underset{\oplus }{\mathop{CH}}\,-\overset{\alpha }{\mathop{C{{H}_{3}}}}\,\)
Six alpha-hydrogens in the above compound.
Type 3: Odd electron conjugation
- \(\overset{\alpha }{\mathop{C{{H}_{3}}}}\,-\overset{\bullet }{\mathop{C{{H}_{2}}}}\,\)
Three alpha-hydrogens in the above compound.
- \(C{{H}_{3}}-\overset{2\alpha }{\mathop{C{{H}_{2}}}}\,-\overset{\bullet }{\mathop{C}}\,H-\overset{3\alpha }{\mathop{C{{H}_{3}}}}\,\)
Five alpha hydrogens in the above compound.
Applications:
Hyper conjugation is generally used to compare stability of alkene.
No. of Hyperconjugative structures = 1 + No. of alpha hydrogens.
No. of alpha hydrogens is proportional to the No. of Hyperconjugative
Structures:
No. of α – H µ Stability
2. Stability of carbocations:
\(\underset{0\alpha \,-H}{\overset{\oplus }{\mathop{\underset{{}}{\overset{{}}{\mathop{C{{H}_{3}}}}}\,}}}\,\,<\,\underset{3\alpha \,-H}{\overset{\oplus }{\mathop{\underset{{}}{\overset{{}}{\mathop{C{{H}_{2}}}}}\,}}}\,-\,C{{H}_{3}}\,<\,\underset{6\alpha \,-H}{\overset{+}{\mathop{\underset{{}}{\overset{{}}{\mathop{C{{H}_{3}}-\,CH\,-\,C{{H}_{3}}}}}\,}}}\,\)
3. Stability of free radicals:
\(\underset{0\alpha \,-H}{\overset{\bullet }{\mathop{\underset{{}}{\overset{{}}{\mathop{C{{H}_{3}}}}}\,}}}\,\,<\,\underset{3\alpha \,-H}{\overset{\bullet }{\mathop{\underset{{}}{\overset{{}}{\mathop{C{{H}_{2}}}}}\,}}}\,-\,C{{H}_{3}}\,<\,\underset{9\alpha \,-H}{\overset{\bullet }{\mathop{\underset{{}}{\mathop{\underset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{{}}{\mathop{C{{H}_{3}}-\,CH\,-\,C{{H}_{3}}}}}\,}}\,}}\,}}}\,\,\)Reverse Hyper conjugation:-
Only possible if a halogen is in conjugation with pi- bonds.
\(\alpha \,\,Cl-\underset{\alpha }{\mathop{\underset{{}}{\mathop{\underset{Cl}{\overset{Cl}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,}}\,}}\,-CH=C{{H}_{2}}\)
Three alpha chlorines are in conjugation with the pi bond whenever 3 halogens are in conjugation with pi bond and hence it is known as reverse conjugation.
