Cyclic Process
What is cyclic process?
A process in which a system goes from an initial state to a final state and returns back to the initial state is called a cyclic process. The net energy change in a cyclic process is zero. That means, in a cyclic process, the system starts and returns to the same thermodynamic state.
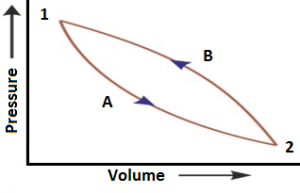
Let consider that a system changes from state A to state B, and then back to state A again. For the change from A to B, ΔU₁ = UB – UA and for the change from B to A, ΔU₂ = UA – UB.
The total energy change ΔUTotal = ΔU₁ + ΔU₂ = (UB – UA) + (UA – UB) = 0.
For a cyclic process, ∆H must also be zero as H is a state function.
Example: The Carnot cycle is the best example of the cyclic process.
The Carnot cycle: The cycle was first discovered by a French engineer Sadi Carnot in 1824 which works on reversible cycle and is known as Carnot cycle.
Carnot employed a reversible cycle to demonstrate the maximum convertibility of heat into work. any fluid can be used to operate the Carnot cycle which is performed in an engine cylinder the head of which is suppose alternatively to be perfect conductor or a perfect insulator of the heat.
