Forces of attraction and repulsion between interacting atoms/molecules other than electrostatic or covalent bonds are called inter molecular forces.
Attractive inter molecular forces: Known as van der Waal’s forces
Types:
1. Dispersion forces/ London forces:
- Force of attraction between temporary dipoles.
- Always attractive.
- Significant only at short distances between interacting particles
- Magnitude depends on polarizability of the particle.
E α 1/r⁶
E = interacting energy.
r = distance between two particles.
2. Dipole – dipole forces:
- Force of attraction between permanent dipoles.
- Stronger than London forces, weaker than ion-ion interaction.
E α 1/r³ —- For stationary polar molecules.
E α 1/r⁶ —- For rotating polar molecules.
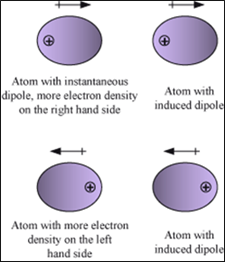
3. Dipole induced dipole forces:
- Acts between a permanent dipole and a non-polar molecule.
- The Permanent dipole of the polar molecule induces dipole on the electrically neutral molecule by deforming its electronic cloud. The non-polar molecule acquires charge.
- Force depends on strength of permanent dipole and polarizability of non-polar molecule.
E α 1/r⁶
4. Hydrogen bonding:
- It is a type of dipole-dipole force.
- Occurs in molecules containing N – H, O – H and H – F bonds (also found in H – Cl bond).
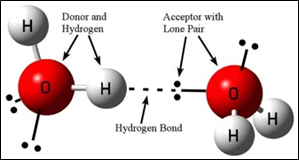
- Strength of the hydrogen bond is depends on the coulombic interaction between the lone-pair electrons of the electro-negative atom of one molecule and the hydrogen atom of another molecule.
Repulsive inter molecular forces:
- Repulsion between the electron clouds and that between the nuclei of two molecules comes into play when particles come in close vicinity of each other.
- Magnitude of the repulsion rises as the distance separating the molecules decreases.
- In liquids and solids the atoms and molecules are very close to each other. Hence the repulsive forces start acting on them. So they are hard to compress.
