Oxides of nitrogen range from N2O (oxidation state of nitrogen +1) through NO, N2O3, NO2, N2O4, N2O5 in which the oxidation state of nitrogen is +5
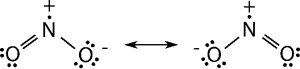
1. NO2– Nitrogen dioxide:
⇒ NO2 is an intermediate in the industrial synthesis of nitric acid.
⇒ This reddish – brown toxic gas has a characteristic sharp, biting odor and is a prominent air pollutant. It absorbs light and leads to the yellow-brown haze sometimes seen hanging over cities.
⇒ It is one of the important components of smog.
⇒ Nitrogen dioxide is a paramagnetic, bent molecule.
⇒ Nitrogen dioxide typically arises via the oxidation of nitric oxide by oxygen in air:
2 NO + O2 → 2 NO2
⇒ In the laboratory, NO2 can be prepared in a two-step procedure where dehydration of nitric acid produces dinitrogen pentoxide, which subsequently undergoes thermal decomposition:
2HNO3 → N2O5 + H2O2
2N2O5 → 4 NO2 + O 2
⇒ The thermal decomposition of some metal nitrates also affords NO2:
2 Pb (NO3)2 → 2 PbO + 4 NO2 + O2
⇒ Alternatively, reduction of concentrated nitric acid by metal (such as copper).
4 HNO3 + Cu → Cu (NO3)2 + 2 NO2 +2 H2O
⇒ Or finally by adding concentrated nitric acid over tin; hydrated tin dioxide is produced as byproduct.
4HNO3 + Sn → H2O + H2SnO3 + 4 NO2
⇒ NO2 exists in equilibrium with the colourless gas dinitrogen tetroxide (N2O4):
2 NO2 ⇌ N2O4
⇒ As suggested by the weakness of the N–O bond, NO2 is a good oxidizer. Consequently, it combusts, sometimes explosively, with many compounds, such as hydrocarbons.
2. N2O – Nitrous oxide:
⇒ Nitrous oxide is commonly known as laughing gas.
⇒ At room temperature, it is a colourless, non-flammable gas, with a slightly sweet odour and taste.
⇒ It is used in surgery and dentistry for its anaesthetic and analgesic effects.
⇒ It is known as “laughing gas” due to the euphoric effects of inhaling it.
⇒ It is also used as an oxidizer in the launching of rockets and in motor racing to increase the power output of engines. At elevated temperatures, nitrous oxide is a powerful oxidizer similar to molecular oxygen.
N ≡ N⁺ – O⁻ ↔ ⁻N = N⁺ = O
3. NO – Nitric oxide:
⇒ Nitric oxide is also known as nitrogen monoxide.
⇒ It is a free radical and is an important intermediate in the chemical industry.
⇒ Nitric oxide is a by-product of combustion of substances as in automobile engines, fossil fuel power plants, and is produced naturally during the electrical discharges of lightning in thunderstorms.
⇒ Nitric oxide (NO) has no colour, odour, or taste and is non-toxic. In the air it is rapidly oxidized to nitrogen dioxide.

